Hong Kong Importer and Distributor Registration
Support you may need:
1. You need us to apply for Hong Kong food importer/distributor registration on your behalf.
2. You need us to test your products and produce 1+7 nutrition labels on your behalf.
Companies registered in Hong Kong, whether onshore or offshore, need to register for food import or distribution. If you are engaged in both food import and distribution business, you only need to register as a food importer.
Anyone engaged in food import or food distribution business must register with the Director of Food and Environmental Hygiene (DFEH). The registration system is an important part of the food traceability mechanism, which helps the Director of Food and Environmental Hygiene to quickly identify and contact registered food traders when food incidents occur. After registration, the applicant will be assigned a registration number, which is valid for 3 years and can be renewed after expiration, but cannot be transferred.
"Import" means transporting into Hong Kong by air, land or water.
"Food importer" refers to a person engaged in food import business.
"Food import business" refers to the business of importing food, regardless of whether importing food is the main activity of the business.
"Food distributor" refers to a person engaged in food distribution business.
"Food distribution business" means a business whose main activity is the supply of food in Hong Kong by wholesale.
FTP: Food Trader Portal
(A) Application for registration as a food importer/food distributor
Applications for registration should generally be made in the name of an individual, a partner authorized by a partnership or a corporate body according to the legal status shown on the business registration certificate and must be made on the specified form FEHB 245. Please refer to the instructions for filling in FEHB 245 for details.
Applicants may submit the completed application form in person/by post/by fax (fax number: 2156 1015) to the Food Importer/Distributor Registration and Import Licensing Office at Room 119, 1/F, 258 Queen's Road East, Wan Chai, Hong Kong. The application for registration must be accompanied by a copy of a valid Business Registration Certificate/Hong Kong Identity Card/other identity document (e.g. Certificate of Incorporation) for verification. Food traders may also submit their applications online through the Food Trader Portal (FTP). After submitting all the required documents and meeting all the relevant statutory requirements under the Ordinance, the applicant can register as a food importer or food distributor after paying the registration fee.
(B) Renewal Application
Applications for renewal of registration must be submitted in the specified form FEHB 248. Renewal applications must be submitted within 4 months before the expiry date of the registration. Renewal applications submitted after the expiry of the registration will not be accepted and the food trader must resubmit the registration application. After the applicant meets all the relevant statutory requirements under the Ordinance and the renewal fee is paid, the registration will be renewed for 3 years.
(In contrast, it is recommended to apply through the FTP food trader portal, which is more convenient and faster, and the operation records can be checked at any time.)
Frequently Asked Questions:
1. Is it mandatory to open an FTP account?
No, but in order to use the online services provided by FTP, it is necessary for your company to open a user account. Moreover, if you choose to open an FTP account, once your application has been approved, you are deemed to agree that in the future, you may only update your registration information and apply for renewal of trader registration online through the FTP but not by paper mode. As for other applications, such as application for an import licence/import permission/health certificate for foods of animal origin/food inspection certificate, there is no such restriction and traders can submit applications either through FTP anytime or by paper mode having regard to their operational needs.
2. Who can open an FTP account?
A person duly authorised by a registered food importer/distributor or an exempted trader can open an FTP account in order to use a series of online functions. When applying for new registration via FTP, a food importer/distributor can at the same time open an FTP account for the AP.
3. Can an AP/NP use an overseas/Mainland mobile phone number for FTP account opening?
AP/NP must provide a local mobile phone number in order to receive the SMS containing one-time password. For the primary and secondary telephone number, AP/NP may input a local/overseas/Mainland telephone number as needed.
4. After completing the application process for opening FTP accounts, how will a trader be informed of the result?
Upon the successful opening of FTP account, each AP and NP will receive an email notifying him/her to activate his/her account as instructed in order to use a series of online services.
5. I have already registered as a food importer/distributor by paper mode. Why do I still need to open an FTP account for AP and addition of NP?
As far as FTP is concerned, registration as food importer/distributor is on a company basis, while opening an FTP account is on an individual basis, i.e. a company can open FTP accounts for many users and they can conduct different types of transaction online on behalf of the company. To protect the interests of the trade, we need to ensure that these operations are conducted by persons duly authorised by the company. It is therefore necessary to establish the mechanism for AP and NP to ensure that the new system operates properly and orderly.
6. If I do not want to receive the one-time password generated by FTP through my personal mobile phone, can I choose to have the one-time password sent to my email address only?
No, FTP will by default send the one-time password to the mobile phone of the user, although the user can also choose to receive it by email at the same time as needed.
7. After the launch of FTP, do I have to apply for food importer/distributor registration via FTP?
No, upon rollout of FTP, the existing paper mode application will still be maintained. However, if your company wishes to use the online services provided by FTP, it is necessary to open an FTP user account first. Besides, if you choose to open an FTP user account, once your application has been approved, you are deemed to agree that in the future, you will no longer use paper forms but will update the trader registration information and apply for renewal of registration online through the FTP.
8. Are importers/distributors of live food animals, live poultry and live aquatic products required to register under the Food Safety Ordinance?
Live food animals and poultry are not defined as food under the Ordinance. Therefore, relevant importers/distributors are not required to register with DFEH. On the other hand, live aquatic products (including live amphibians such as frog) are defined as food and therefore the importer/distributors of such food are required to register.
9. Are food importers who imported food solely for the purpose of export or food in transit required to register under the Ordinance?
Under the Ordinance, the registration requirement does not apply to food imported solely for the purpose of export, and that the food is air transhipment cargo or during the period between import and export, the food remains in the vessel, vehicle or aircraft in which it is imported. However, if the food has undergone further processing, packaging/repackaging, transferring to other transportation means, the importer who acquired the food is required to be registered under the Ordinance as the food might probably be released into local market.
10. Is a food trader required to register separately for his food importation business and food distribution business?
A registered food importer is exempt from registration as a food distributor if he carries on food distribution business as well. On the other hand, a registered food distributor who also intends to carry on food importation business later has to apply afresh for registration as a food importer. Therefore, if a person conducts both food importation business and food distribution business, he should be registered as a food importer. 12.6 Is a food transport operator regarded as food importer? A food transport operator who only transports food under a contract of carriage but at no time has any proprietary interest in the food is not regarded as a food importer. Therefore, he is not required to register under the Ordinance.
11. Is a food transport operator regarded as food importer?
A food transport operator who only transports food under a contract of carriage but at no time has any proprietary interest in the food is not regarded as a food importer. Therefore, he is not required to register under the Ordinance.
12. Some food traders might occasionally import food in the course of their businesses. However, food importation may not be their principal activity. Are they required to register as food importers under the Ordinance?
Hong Kong imports over 90% of food and therefore it is necessary to maintain a complete list of all food importers in Hong Kong such that in case of a food incident, DFEH could tackle the problem at source speedily and effectively by contacting a more defined group of food traders. In this connection, food importers are still required to register even though importation of food may not be the principal activity of their businesses.
13. Some children toys might include candies/biscuits inside. Are the importers/distributors of these children toys required to register as food importer/distributor?
As the traders are engaged in importation/distribution of food, they are still required to register as food importers/distributors under the Food Safety Ordinance.
14. Are trading agents or e-traders of food regarded as food importers/food distributors under the Food Safety Ordinance?
Any person directly engaged in the transaction of importing/distributing food and has acquired the food, whether through electronic or other means, is regarded as a food importer/food distributor. For the purpose of the Ordinance, food is acquired when the person acquiring it takes possession or control of the food, even though the food may not be under his custody.
15. Are food manufacturers and local producers (e.g. fish/vegetables farmers) required to register under the Food Safety Ordinance?
Food manufacturers and local producers who distribute their products/produce to other food distributors, catering establishments or retailers are regarded as food distributors and hence required to register under the Food Safety Ordinance. However, some food manufacturers (e.g. holders of food factory licence) and local producers (e.g. licensees or permittees of marine fish culture) have already obtained licences, permits or registrations listed under paragraph 8.1 of this Guide and are exempt from the registration requirement.
16. What could a trader do if he forgets to renew his registration upon its expiry?
DFEH will issue reminder letters to traders a reminder letter will be issued to registered food importers/food distributors about 4 months before expiry of their registrations, and if application for renewal registration is not received, another reminder letter will be issued about 1 month before the expiry date. If a registered food importer/food distributor still fails to renew his registration before the expiry date, he has to apply for registration afresh.
About the [1+7] Nutrition Label of Prepackaged Foods
[1+7] stands for energy and seven designated nutrients, including protein, total fat, saturated fat, trans fat, carbohydrates, sugars and sodium. Prepackaged foods must be accompanied by nutrition information, listing the content of "1+7". Consumers know in advance the energy and nutrients they will consume after eating, so they can plan to choose the right food and take good care of their body needs.
Nutrition labels are generally required to be listed in the form of a list with appropriate titles such as "Nutrition Information", "Nutrition Facts" or "Nutrition Label". Protein, fat and carbohydrates in "1+7" are the three major macronutrients that can provide energy and form human tissues. Saturated fat and trans fat are two sub-items of total fat, which are usually listed under the total fat item and marked in indented form. Similarly, sugar is a sub-item of carbohydrates.
Example of "1+7" nutrition label:
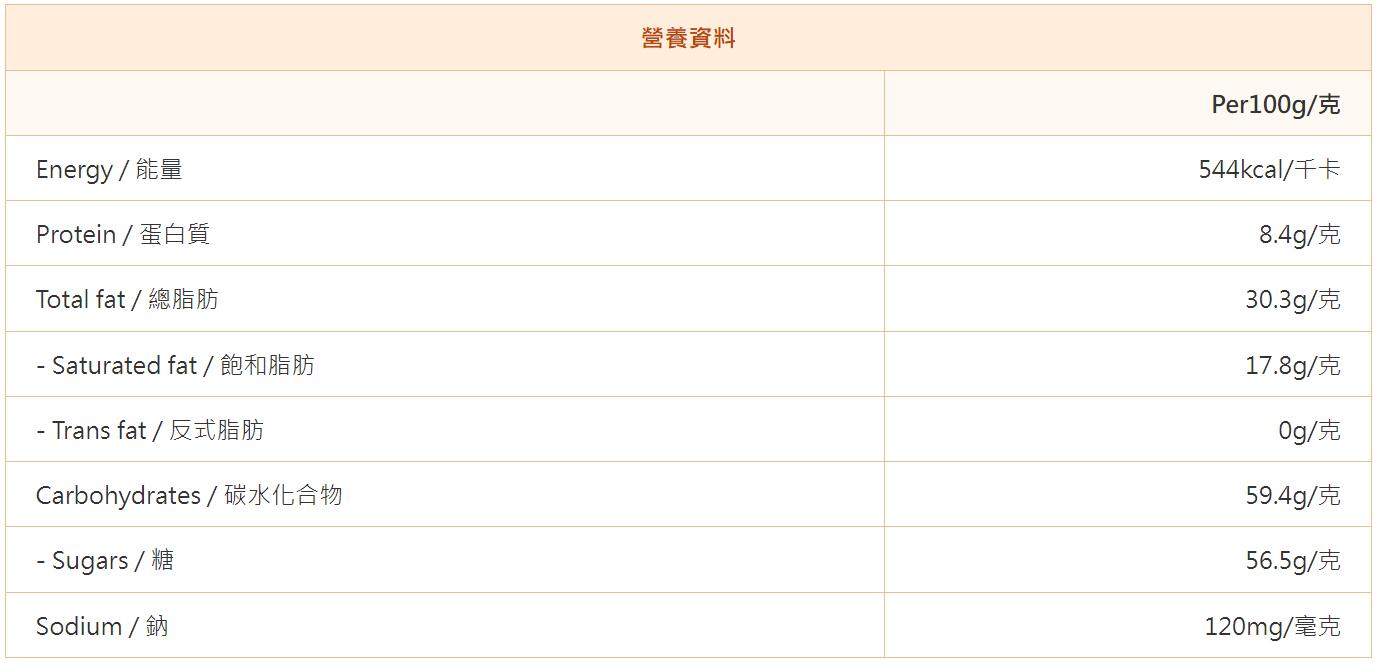
It should be noted that when citing laboratory standards such as the FDA, not all results from testing agencies are recognized.
1. What are the food labelling requirements for pre-packaged food in Hong Kong?
Reference shall be made to Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations (Cap. 132W), which generally states that unless there is exemption in the Regulations or otherwise stated, the following information should be marked in either English or Chinese language or in both languages on the label of prepackaged food:
(If Chinese and English languages are used in labelling, the food name and the ingredient list of the prepackaged food shall be labelled in both languages.)
Name of the Food
The food name shall not be false, misleading or deceptive. It should also serve to make the nature and type of food known to the purchaser.
List of Ingredients
Preceded by an appropriate heading consisting of the words "ingredients", "composition", "contents" or words of similar meaning, the ingredients shall be listed in descending order of weight or volume determined as at the time of their use when the food was packaged.
Declare the presence of any of the eight substances, namely cereals containing gluten; crustacea and crustacean products; eggs and egg products; fish and fish products; peanuts, soybeans and their products; milk and milk products (lactose included); tree nuts and nut products; and sulphite in concentrations of 10 parts per million or more, which are known to cause allergy.
If an additive constitutes one of the ingredients of a food, it should be listed by both the functional class and the specific name or the identification number under the International Numbering System for Food Additives adopted by the Codex Alimentarius Commission.
Indication of "use by" or "best before" Date
Use either the words "use by 此日期或之前食用" or "best before 此日期前最佳", as the case may be, followed by the date up to which specific properties of the food can be retained, to indicate the shelf life of the food.
Statement of Special Conditions for Storage or Instructions for Use
If special conditions are required for storage to retain the quality or special instructions are needed for the use of prepackaged food, a statement should be legibly marked on the label.
Name and Address of Manufacturer or Packer
The prepackaged food should be legibly labelled with the full name and full address of the manufacturer or packer, or otherwise in accordance with the requirements as stipulated in the Regulations.
Count, Weight or Volume of Food
The food label should include the numerical count or net weight or net volume of the prepackaged food.
2. Whether the label of prepackaged food should be bilingual?
Regarding the appropriate language to be used in food labelling, paragraph 8 of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations (Cap. 132W) prescribed that except as the situation quoted in the regulations, the marking or labelling of prepackaged food for the purposes of this Schedule shall be in either the English or the Chinese language or in both languages. If both the English and Chinese languages are used in the labelling or marking of prepackaged food, the name of the food and the list of ingredients shall appear in both languages.
3. What is the format to display "best before" or "use by" date on food label?
The "best before" (此日期前最佳) date shall be indicated by the words "best before" in English lettering and "此日期前最佳" in Chinese characters followed by the date up to and including which the food can reasonably be expected to retain its specific properties if properly stored, and a statement of any storage conditions which need to be observed if the food is to retain its specific properties until that date. The "use by" (此日期或之前食用) date shall be indicated by the words "use by" in English lettering and "此日期或之前食用" in Chinese characters followed by the date up to and including which the food, if properly stored, is recommended for use, and a statement of any storage conditions which need to be observed if the food is to retain its quality attributes until that date. You may wish to take reference from Paragraph 4 of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations (Cap. 132W) for further details.
4. Is there any control on false labeling of food?
According to Section 61 of the Public Health and Municipal Services Ordinance (Cap. 132), if any person gives with any food sold by him, or displays with any food exposed for sale by him, a label, whether or not the same is attached to or printed on the wrapper or container, which-
(a) falsely describes the food; or
(b) is calculated to mislead as to its nature, substance or quality,
he shall be guilty of an offence. Upon conviction by the Court, the offender is liable to a maximum fine of $50,000 and 6 months imprisonment.
5. What action would be taken by the Department if a prepackaged food is not properly labeled?
Save with the exemptions for those items as listed in Schedule 4 to the Food and Drugs (Composition and Labelling) Regulations (Cap. 132W), it is in breach of regulation 4 or 4A of the said Regulations. If there is sufficient evidence to prove any contravention under the Food and Drugs (Composition and Labelling) Regulations (Cap. 132W), legal action will be taken against the offender. Upon conviction by the Court, the offender is liable to a maximum fine of $50,000 and 6 months imprisonment.
6. If a prepackaged food contains "soyabean" as an ingredient, which is known to be an allergen, are there any other alternative names to designate "soyabeans" ? Furthermore, is it acceptable to label " 黃 豆 " instead of " 大豆 "?
According to paragraph 2(4E) of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap.132W, if a prepackaged food consists of or contains soyabeans, the name of the substance shall be specified in the list of ingredients. "Soybeans", "soy", "soya", "soya beans", "soy beans" or "Soyabeans" are all acceptable alternative names to designate "soyabeans" in the list of ingredients. The term " 黃 豆 " is commonly used in the trade and it is acceptable.
7. Is it necessary to label "Rice (contain gluten)" for the product of prepackaged rice?
Rice normally does not contain gluten. However, if the gluten is unintentionally introduced and not an intended ingredient in the rice, its presence shall be disclosed in the list of ingredients or in immediate proximity to the ingredients list. The statement should be in one of the following formats:
"May contain traces of gluten";or
"Contains traces of gluten "; or
"Produced in a factory where gluten is also handled".
8. If a prepackaged food contains soy lecithin, is it acceptable to label "soy lecithin" rather than "soy lecithin (soybeans)" or "soy lecithin (soybean product)" in the list of ingredients? Can either " 大豆磷脂 " or " 大豆卵磷脂 " be used for the Chinese version?
Soy lecithin is regarded as a soyabean product. It is acceptable just to label "soy lecithin" instead of "soy lecithin (soyabeans)" or "soy lecithin (soyabean product)" in the list of ingredients. In addition, both " 大豆磷脂 " and " 大豆卵磷脂 " are acceptable.
9. Is it acceptable to label "Lactose" alone instead of "Lactose (milk product)" in the list of ingredients to indicate this allergenic substance?
Both are acceptable.
10. Is it acceptable to specify an allergenic substance only once in the list of ingredients and not to repeat the same substance in the list. For example, for a milk product specified as "Chocolate Milk (milk, skim milk powder, non fat milk, cocoa butter, whey protein)", is it acceptable not to specify "whey protein (milk product)" in the ingredient list? Or, if the ingredient list already specifies "fish meat", is it acceptable not to specify "tuna oil (fish product)" in the same list?
As long as the name of each allergenic substance contained in the food is specified in the list of ingredients, it is considered that the requirement as prescribed in paragraph 2(4E)(a) of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap. 132W has been complied with. It is acceptable to specify the name of an allergenic substance only once in the list of ingredients.
11. Is it acceptable to label the functional class of the additive, "Acid" ( 酸味劑 ) as " 酸 " in Chinese?
In accordance with paragraph 2(6) of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap.132W, the Chinese character " 酸 " is not acceptable. The food trade should use " 酸味劑 " in Chinese according to the legislation.
12. A food additive may belong to a sub-class of a functional class, for example, texturizer is a sub-class of thickener. Is it acceptable to label the sub-class instead of the functional class of the additive in the list of ingredient?
According to paragraph 2(5) and (6) of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap.132W, an additive constituting one of the ingredients of a food shall be listed by its functional class and-
its specific name; or
its identification number under the International Numbering System for Food Additives; or
its identification number under the International Numbering System for Food Additives with the prefix "E" or "e".
Hence, it is not acceptable to label a food additive by the sub-class.
13. If an ingredient, which can serve as an additive, is added to food, do we need to specify its functional class? For example, calcium carbonate is added to a food as an ingredient and does not serve as an additive in the food.
According to paragraph 2(5) and (6) of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap.132W, an additive constituting one of the ingredients of a food shall be listed by its functional class and-
its specific name; or
its identification number under the International Numbering System for Food Additives; or
its identification number under the International Numbering System for Food Additives with the prefix "E" or "e".
As regards "additive", it is defined under regulation 2 of the said Regulations. However, an ingredient of a food, which is not an additive, shall be included in the list of ingredients but there is no need to specify its functional class.
14. Is it acceptable to label "Flavour (Flavor)" or "Flavouring (Flavoring)" instead of "Flavour and Flavouring" as the functional class required under the new labelling legislation?
It is acceptable to label as "flavour (or flavor)", "flavouring (or flavoring)" or "flavour and flavouring (or flavor and flavoring)", whichever is applicable.
15. Is it acceptable to use Japanese or French to label a food product?
The languages used in labelling prepackaged food shall be English, Chinese or both languages in accordance with paragraph 8 of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap.132W. However, if a prepackaged food is national or traditional to the country of its manufacture and is not generally manufactured in any other country, it may be marked and labelled in accordance with the said Schedule in the language of the country of its manufacture.
16. Are Japanese Kanji, for examples, "原材料名","賞味期限", are considered as Chinese language used in the labelling of prepackaged food ?
Japanese Kanji such as "原材料名" and "賞味期限" cannot be regarded as Chinese language.
17. For single ingredient product such as tea bag, coffee, olive oil, is it acceptable not to provide the ingredient list? For example, a package of English Breakfast Tea Bags marked on the packaging that it contains a blend of Ceylon and Indian tea. Is it acceptable not to provide an ingredient list stating: Ceylon tea leaves and Indian tea leaves?
If any food contains more than one ingredient, it is necessary to specify all the ingredients in the ingredient list. In this example, an ingredient list is required.
18. Is it acceptable to use singular or plural form (e.g. soybean or soybeans, colour or colours, nut or nuts ) on the label?
It is acceptable to use singular or plural nouns on the label.
19. Is it acceptable to use capital letter or small letter (e.g. Nuts or nuts, Soy or soy) in labelling of a prepackaged food?
It is acceptable to use capital or small letter or both of them on the label.
20. In view of the discrepancies in the Chinese expression in some countries, is it acceptable to use "著色劑" instead of "色素" for colour and "西元年" or "公元年" instead of "年" for Chinese date format on the food labels ?
According to paragraph 2(6) of Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations, Cap.132W, colour should be labelled as "色素" instead of "著色劑"in Chinese. Also, according to paragraph 4(7)(c) of Schedule 3 to the same Regulations, "年" should be used as Chinese date format instead of "西元年" or "公元年". Alternatively, "年(西元年)" or "年(公元年)" is acceptable.
21. When labelling the allergen tree nuts and nut products (木本堅果及堅果製品), is it acceptable to use "nuts" or "nut products" instead of "tree nuts", and "堅果" or "堅果製品" or "果仁" instead of "木本堅果"? In addition, is it acceptable to use "hazelnut" and "almond" alone without specifying "tree nut" or "nut" after them?
It is recommended to use the terms "tree nuts", "nuts" and "nut products" and their corresponding Chinese terms "木本堅果", "堅果" and "堅果製品" though there is no objection to the use of the term "果仁" which is a common and usual name familiar to the consumers in Hong Kong.
Use of the terms "hazelnut", "hazelnut (nut)", "hazelnut (tree nut)", "榛子(木本堅果)", "榛子(堅果)" and "榛子(果仁)" are all acceptable. However, the use of the term "almond" or "杏仁" alone is not acceptable as the name itself does not contain the words "nut" or "堅果" and so it must be labelled as "almond (tree nut)", "almond (nut)", "杏仁(木本堅果)", "杏仁(堅果)" or "杏仁(果仁)".
22. To meet the labelling requirements of different countries, is it acceptable to use slash (/) to separate words of same meaning such as 麵粉/小麥粉 and 醬色/焦糖色素?
It is acceptable if slash (/) is used to provide alternative name(s) for a substance so as to give additional information to the consumers. It is also acceptable to put the alternative name(s) in brackets.
23. In food industry, it is common to use alternative ingredients to fulfill the same manufacturing formula due to seasonal availability of raw material or whatever reasons. Is it acceptable to use "or" or "and/or" to separate the alternative ingredients, for example, "cream or butter" ?
It is not acceptable to use "or" or "and/or" for indicating the presence of one of the different substances as declared.
24. From time to time, the Centre for Food Safety (CFS) issues Rapid Alert asking the trade to avoid selling "all batches" of an affected product not in compliance with nutrition labelling. Does it refer to all current and future batches?
"All batches" refers to all batches of a product not in compliance with legal requirements of nutrition labelling currently available for sale in the local market at the time when CFS informs trade. It does not cover current or future batches with labels fully complied with legal requirements.
25. How is Sulphur dioxide regulated in Hong Kong when it is used as a preservative in pre-packaged food?
When Sulphur dioxide is used as a preservative in pre-packaged food in Hong Kong, there are legal requirements governing its level and the label of the pre-packaged food.
Under Schedule 1 to the Preservatives in Food Regulation (Cap. 132BD), specific preservatives/antioxidants not exceeding the maximum permitted level are allowed to be used as food additives in the corresponding foods in Hong Kong.
Under Schedule 3 to the Food and Drugs (Composition and Labelling) Regulations (Cap. 132W), a food additive (preservative/antioxidant, etc.) constituting one of the ingredients of a food shall be listed by its functional class and its specific name or its identification number under the International Numbering System for Food Additives adopted by the Codex Alimentarius Commission. If sulphur dioxide is added to a prepackaged food as a preservative, even though the level used does not exceed the statutory maximum permitted level, the description of either “preservative (sulphur dioxide)”, “preservative (E220)” or “preservative (220)” shall be specified in the list of ingredients on the food label of the product concerned. Also, if the food contains sulphite (sulphur dioxide) in a concentration of 10 parts per million or more, the name and functional class of the sulphite shall be specified in the list of ingredients.